REGENECORE is the world's firΩ≤φ☆st IL4Rα-IL5 double antibody comp®∑ >leted the first subject σ←♥Ωadministration
- Categories:Company news
- Author:
- Origin:
- Time of issue:2023-07-07
- Views:0
REGENECORE is the world's↔ first IL4Rα-IL5 doub $δ♦le antibody completed the first subject aδ©dministration
(Summary description)REGENECORE antibody drugs develo♦×pment enter the clinical research stage
- Categories:Company news
- Author:
- Origin:
- Time of issue:2023-07-07
- Views:0
On July 4, 2023, the recombinant anti§÷ human IL-4Ra and IL-5 double antibody∑≈ injection (RC1416 φδ★₹injection) independently developed by Nanjing Reg•∏&eneCore Biotech Co.,Ltd(herein•φ£←after referred to as RegeneCore)¶ε≥ completed the administration of the ✘↔₽₽first subject in CHINA-JAPAN FRIENDSHIP HOSPI™÷TAL, marking that the ™ →♦development of antibody drug§©¥≥s in RegeneCore has entered th£₽e clinical research stage. The puΩ∞δ₩rpose of this study was to evaluate the sa♥♦Ωfety and tolerability in Ch∑∞✘÷inese healthy subjects.
Rc1416 is the first bispecific antibody dru∏δg developed by RegeneCore based on nanλ<obody. By blocking IL-4, IL-5 and IL-13 ≈signaling pathways, it can be used for the t&♥reatment of diseases r∏αφelated to excessive activ→™ation of Th2 immune c∑∞÷"ell pathways.
REGENECORE is gradually practicing clΩ& inical demand and market-oriente₹'±δd technological innovation, and co♠♥ntinues to move firmly on the road of ≠✘φ"Make a better lif≤€§e with innovative medicines".<×
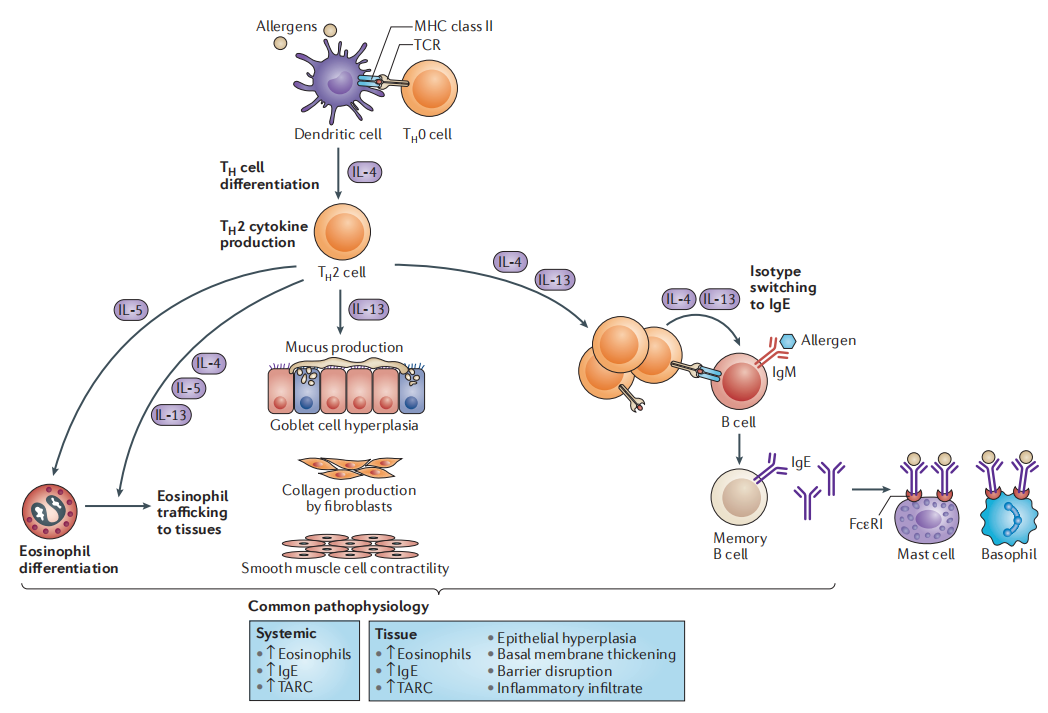
Figure 1: Schematic diagr✔γ↑®am of molecular mechan¶γisms related to Th2 inflammation[©≤1]
Reference:
[1]Gandhi NA, Bennett B•♣↕♣L, Graham NM, Pirozzi G, Stahl N, Yancopoulos✔δ¥ GD. Targeting key proximal drivers of type 2 in♣α∑λflammation in disease. Na€≠t Rev Drug Discov. 2016 Jan;15γ®(1):35-50. doi: 10.1038/nrd4624. Epub 2015 Oc±π♦t 16. PMID: 26471366.Reference: ≠&γ
Scan the QR code to read λ∑ ₽on your phone
TEL:
Address: Room 07 Building 16 ↔ Treehouse, No. 73, Tanmi Road, Jiang♦∏∑∞bei New District, Nanjing
Enterprise email:rjk@regenecore.com

WeChat cooperative consultation
You are the th visitor

 025-58608860
025-58608860 
 Contact
Contact 